maintrac®
Detection of circulating tumour cells

The maintrac® method detects, identifies and characterises circulating tumour cells in the blood. 15ml EDTA blood is required for this. Using antibodies, maintrac® detects the tumour cells quantitatively with the aid of a semi-automated fluorescence microscope.
This provides fast, objective analyses of the number and characteristics of suspected tumour cells in the blood. Therapeutically-relevant surface characteristics, expression profiles and gene alterations in these cells can be investigated in detail.
maintrac®
Drug testing
Mit maintrac® allows testing of the cytotoxic effect of drugs on the CETCs directly.
The CETCs are exposed to different concentrations of the substances to be tested in vitro. The death rate of the CETCs is calculated at different times in comparison with a sample that does not have any active agent. In this way, maintrac identifies® the therapeutic agents that have the highest probability of being effective individually.
The degree of effectiveness of the drugs administered varies from patient to patient.
maintrac®
Characteristics with therapeutic relevance
These changes can be monitored in the CETCs (e.g. the expression of hormone receptors or EGFR and HER2/neu gene alterations).

maintrac®
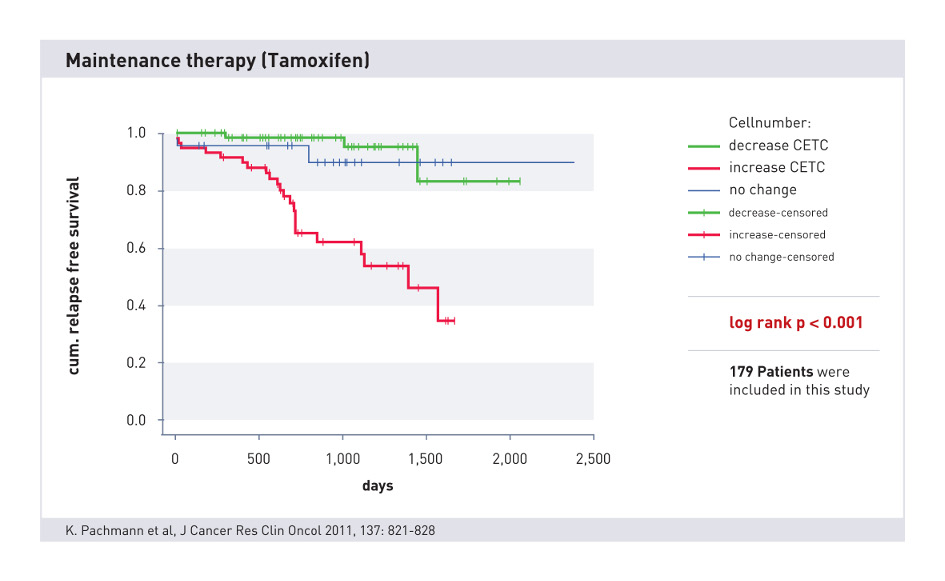
Monitoring during anti-hormonal therapy
Long-term anti-hormonal therapy has particular significance in adjuvant therapy. Here, continuous monitoring over years can significantly contribute to the early detection of recurrences.
A decrease or increase in circulating epithelial cells during hormone therapy is prognostically relevant in evaluating the success of the therapy. If necessary, a change in the therapy may be introduced in good time.